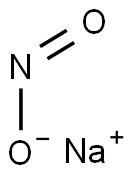
Sodium nitrite synthesis
- Product Name:Sodium nitrite
- CAS Number:7632-00-0
- Molecular formula:NaNO2
- Molecular Weight:69
7631-99-4
10 suppliers
$10.00/5g

7664-41-7
456 suppliers
$15.00/100ml
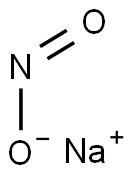
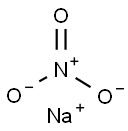
7632-00-0
809 suppliers
$14.00/25g
Yield:-
Reaction Conditions:
with sodium hydroxide for 2 h;Electrolysis;Time;
Steps:
2.3. Electrochemical tests in cathodic reactions
Ferricyanide reduction was studied with polished Au and porousCu-Ni RDEs in deaerated solutions containing 20 mM K3Fe(CN)6,20 mM K4Fe(CN)6and 1 M KOH. Experiments of nitrate reduc-tion were performed in a two-compartment deaerated cell, with100 ml of 1 M NaOH and 0.1 M NaNO3in the working compartment.Compact and porous Cu-Ni RDEs were used for recording cyclicvoltammograms and chronoamperometries, compact and poroussheet electrodes (about 3.0 cm2of immersed area) for prolongedelectrolyses. Analyses of the products were performed by with-drawing 0.1 mL samples from the cathodic compartment with aprecision pipette and assessing the products with a Metrohm model850 Professional IC Ion Chromatograph [38].
References:
Mattarozzi, Luca;Cattarin, Sandro;Comisso, Nicola;Gambirasi, Arianna;Guerriero, Paolo;Musiani, Marco;Vázquez-Gómez, Lourdes;Verlato, Enrico [Electrochimica Acta,2014,vol. 140,p. 337 - 344]

7631-99-4
10 suppliers
$10.00/5g
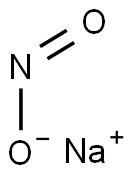
7632-00-0
809 suppliers
$14.00/25g
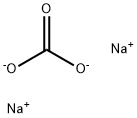
497-19-8
1301 suppliers
$14.00/1KG
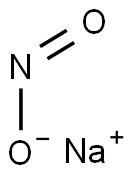
7632-00-0
809 suppliers
$14.00/25g

7631-99-4
10 suppliers
$10.00/5g

7727-37-9
101 suppliers
$323.00/56l

7664-41-7
456 suppliers
$15.00/100ml
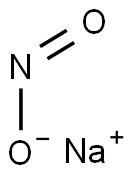
7632-00-0
809 suppliers
$14.00/25g

7664-41-7
456 suppliers
$15.00/100ml
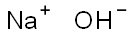
1310-73-2
1260 suppliers
$14.00/25g
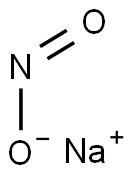
7632-00-0
809 suppliers
$14.00/25g