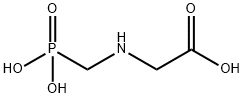
글리포세이트
|
|
글리포세이트 속성
- 녹는점
- 230 °C (dec.) (lit.)
- 끓는 점
- 465.8±55.0 °C(Predicted)
- 밀도
- 1.74
- 인화점
- 230°C
- 저장 조건
- APPROX 4°C
- 용해도
- DMSO: slightly soluble; PBS (pH 7.2): slightly soluble
- 물리적 상태
- solid
- 산도 계수 (pKa)
- 1.22±0.10(Predicted)
- 냄새
- odorless
- 수용성
- 1.2 g/100 mL
- 분해온도
- 230 ºC
- Merck
- 13,4525
- BRN
- 2045054
- 안정성
- Stable. Incompatible with metals, strong oxidizing agents, strong bases. May be light sensitive.
- InChIKey
- XDDAORKBJWWYJS-UHFFFAOYSA-N
- LogP
- -2.360 (est)
- CAS 데이터베이스
- 1071-83-6(CAS DataBase Reference)
- IARC
- 2A (Vol. 112) 2017
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,N,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 41-51/53-62-37/38-36/37/38-36-22 | ||
| 안전지침서 | 26-39-61-2-37-36 | ||
| 유엔번호(UN No.) | UN 3077 9/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | MC1075000 | ||
| HS 번호 | 29319090 | ||
| 유해 물질 데이터 | 1071-83-6(Hazardous Substances Data) | ||
| 독성 | LD50 in rats, mice (mg/kg): 4873, 1568 orally (Bababunmi) | ||
| 기존화학 물질 | KE-28510 | ||
| 중점관리물질 필터링 | 별표2-84 | ||
| 암, 돌연변이성물질 필터링 | 350 |
글리포세이트 C화학적 특성, 용도, 생산
개요
Glyphosate (N-(phosphonomethyl)glycine; 1071-83-6) is the active ingredient in several commercial herbicides for nonselective weed control. Glyphosate herbicides are among the world’s most widely used herbicides. Roundup?, containing the active ingredient glyphosate, was developed and introduced by Monsanto Company in 1974. Other formulations include WeatherMax, UltraMAX, Buccaneer, Razor Pro, Rodeo, and AquaMaster?. Some crops such as soybeans and cotton have been genetically engineered to be resistant to glyphosate (Roundup Ready), allowing farmers to use glyphosate as a postemergence herbicide. The United States Environmental Protection Agency (EPA) considers glyphosate to be relatively low in toxicity compared to organochlorine and organophosphate pesticides.화학적 성질
Glyphosate is a broad-spectrum, non-selective systemic herbicide. It is a colorless crystal at room temperature and is soluble in acetone, ethanol, xylene, and water. Glyphosate is used for the control of annual and perennial plants, including grasses, sedges, broadleaved weeds, and woody plants. It can be used on non-cropland as well as on many varieties of crops. Glyphosate itself is an acid, but it is commonly used in salt form, most commonly isopropylamine salt. It may also be available in acidic or trimethylsulfonium salt forms. It is generally distributed as water-soluble concentrates and powders. Glyphosate is a GUP.용도
Glyphosate is the active ingredient in several commercial herbicides. It is a broad-spectrum systemic herbicide for various types of weeds, grasses (Poaceae), and woody plants.일반 설명
Odorless white powder. Decomposition begins at approximately 419°F (darkens). pH (1% solution in water) 2.5.반응 프로필
Glyphosate may react with galvanized steel or unlined steel (except stainless steel) containers to produce hydrogen gas which may form a highly combustible or explosive gas mixture. Glyphosate can react with caustic (basic) materials to liberate heat. Glyphosate is corrosive to iron.건강위험
Glyphosate is practically non-toxic if ingested, with a reported acute oral LD50 of 5600 mg/kg in the rat. The toxicities of the technical acid (glyphosate) and the formulated product (Roundup) are nearly the same. Laboratory animals, such as rats, dogs, mice, and rabbits, exposed to glyphosate for 2 years did not indicate any kind of adverse health effects.화재위험
Flash point data for Glyphosate are not available; however, Glyphosate is probably combustible.Pharmacology
Glyphosate is the only known inhibitor of the biosynthesis of aromatic acids that has been commercialized as a successful herbicide (1). Glyphosate acts as a competitive inhibitor of phosphoenolpyruvate, the natural substrate of the enzyme 5-enolpyruvyl-shikimate-3-phosphate (EPSP) synthase, and causes amassive accumulation of shikimate in treated plant tissue (1).Glyphosate is a nonselective herbicide, and it has been characterized as a low-risk herbicide for the evolution of herbicide resistance. A few weed species are somewhat tolerant to glyphosate, probably due to uptake or translocation mechanisms, but no plant species has sufficient resistance to glyphosate to allow its use directly on the crop as a selective herbicide. The complicated procedure used to genetically engineer the commercialized glyphosate-tolerant crops (31) would suggest that the evolution of glyphosate-resistant weeds will be a very slow process and that the level of resistance from field selection will be relatively low.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Human systemic effects: arrhythmias, blood pressure lowering, body temperature increase, change in heart rate, convulsions, darrhea, fibrosing alveolitis, fibrosis, hypermoultty, respiratory depression, respiratory stimulation. Used as an herbicide. When heated to decomposition it emits very toxic fumes of NOx and POx.잠재적 노출
A potential danger to those involved in the manufacture, formulation, and application of this nonselective and nonresidual pre-emergence organophos phate herbicide. Has wide residential use in the United States for the control of weeds.신진 대사 경로
The photolytic degradation of glyphosate results in the formation of glycine, (aminomethyl)phosphonic acid (AMPA), and NH3. Glyphosate undergoes nitrogen ? carbon cleavage on reaction with m- chloroperoxybenzoic acid, leading ultimately to many of the same products formed on their metabolism and environmental degradation. It is suggested that insoluble complexes of glyphosate with iron(III), copper(II), calcium, and magnesium ions are formed at near-neutral pH, a mechanism of which is the inactivation of glyphosate in contaminated groundwater.268 The bacterium degrades high levels of glyphosate, primarily by converting to AMPA. Appreciable uptake of glyphosate is observed with seedlings and leaves and to a lesser extent with culture cells in the form of non-metabolized glyphosate, with AMPA as the only detectable metabolite.신진 대사
In soils, glyphosate is rapidly mineralized within 1 to 2 weeks, and degradation occurs under aerobic and anaerobic conditions (79). The C?P bond is relatively resistant to chemical degradation, but several bacteria, e.g., Arthrobacter (80), Pseudomonas (81), various members of the Rhizobiaceae family (82), and certain fungi (83), have been shown to metabolize glyphosate.운송 방법
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz ardous material, Technical Name Required.비 호환성
Organophosphates are susceptible to for mation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water, and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithio carbamates, isocyanates, mercaptans, nitrides, sulfides (releasing heat, toxic, and possibly flammable gases), thio sulfates, and dithionites (releasing hydrogen sulfate and oxides of sulfur). Solutions are corrosive to iron, unlined steel, and galvanized steel, forming a highly combustible or explosive gas mixture. Do not store glyphosate in contain ers made from these materials.글리포세이트 준비 용품 및 원자재
원자재
Iminodiacetic acid
암모니아수
클로로아세트산
Dimethyl ester
과산화 수소
메틸아민
포르말린
Para-포름알데하이드
글리신
트라이에틸아민
다이에탄올아민
삼염화 인
아인산
트리메틸포스파이트
N-(Carboxymethyl)-N-(phosphonomethyl)-glycine
시안화수소
염산
3-Amino-4-chlorobenzenesulfonic acid
메틸알콜
수산화나트륨
Aqueous agent
메틸포스폰산
이미노 디초산
헥사민
SURFACTANT
다이메틸 히드로겐 포스피트
황산
수산화칼슘
암모니아(가스)
준비 용품
글리포세이트 공급 업체
글로벌( 593)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| AS WATER CO., LTD. | +86-18994963526 +8618994963526 |
1346073549@qq.com | United Kingdom | 173 | 58 |
| Hebei Crovell Biotech Co Ltd | +86-19930503285 |
zoey@crovellbio.com | China | 5740 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29811 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21975 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
fandachem@gmail.com | China | 9378 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2990 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 |
kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29922 | 58 |
| HEBEI YINGONG NEW MATERIAL TECHNOLOGY CO.,LTD | +86-19933070948 +86-19930599843 |
claire@hbyingong.com | China | 945 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | CHINA | 2933 | 58 |