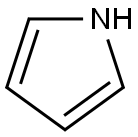
피롤
|
|
피롤 속성
- 녹는점
- -23 °C (lit.)
- 끓는 점
- 131 °C (lit.)
- 밀도
- 0.967 g/mL at 25 °C (lit.)
- 증기 밀도
- 2.31 (vs air)
- 증기압
- 8.7 hPa (20 °C)
- FEMA
- 3386 | PYRROLE
- 굴절률
- n
20/D 1.508(lit.)
- 인화점
- 92 °F
- 저장 조건
- Store at +2°C to +8°C.
- 용해도
- 60g/l
- 산도 계수 (pKa)
- 15(at 25℃)
- 물리적 상태
- Liquid
- 색상
- Clear almost colorless to brownish
- 수소이온지수(pH)
- >6 (10g/l, H2O, 20℃)
- 냄새
- at 0.10 % in propylene glycol. sweet warm nutty ethereal
- ?? ??
- nutty
- 폭발한계
- 3.10-14.8%(V)
- 수용성
- 60 g/L (20 ºC)
- 감도
- Air & Light Sensitive
- Merck
- 14,8014
- JECFA Number
- 1314
- BRN
- 1159
- 안정성
- Stable. Incompatible with strong acids, strong oxidizing agents. Combustible.
- InChIKey
- KAESVJOAVNADME-UHFFFAOYSA-N
- LogP
- 0.85
- CAS 데이터베이스
- 109-97-7(CAS DataBase Reference)
- NIST
- Pyrrole(109-97-7)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-20-25-41 | ||
| 안전지침서 | 26-37/39-45-39-24-16 | ||
| 유엔번호(UN No.) | UN 1992 3/PG 3 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | UX9275000 | ||
| F 고인화성물질 | 8-10-23 | ||
| 자연 발화 온도 | 550 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29339900 | ||
| 유해 물질 데이터 | 109-97-7(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 137 mg/kg | ||
| 기존화학 물질 | KE-29973 |
피롤 C화학적 특성, 용도, 생산
화학적 성질
Six π-electrons are distributed over the five ring atoms of pyrrole. Delocalization of these electrons stabilizes the ring and the lone pair of electrons on the nitrogen atom, which is responsible for the usual basicity of nitrogen compounds, is involved in the electron cloud, and is not available for sharing. Hence, pyrrole is an extremely weak base and the pyrrolic nitrogen is not readily susceptible to electrophilic enzymic attack (Damani, 1985). There is a high electron density, however, at all positions of the ring, which causes pyrrole to be reactive toward electrophilic substitution. In general, electrophilic substitution reactions on the neutral molecule occur preferentially at the C-2 or C-5 positions (Jones and Bean, 1977; Damani and Crooks, 1982).물리적 성질
Pyrrole is a colorless to brown liquid that has a sweet, warm-ethereal smell, similar to chloroform. It dissolves in ethanol, ether, benzene, dilute acids, and most non-volatile oils but does not dissolve in water or dilute alkalis. When stored for extended periods, it tends to aggregate and become brown due to the influence of light.정의
ChEBI: 1H-pyrrole is a tautomer of pyrrole that has the double bonds at positions 2 and 4. It is a pyrrole and a secondary amine. It is a tautomer of a 2H-pyrrole and a 3H-pyrrole.제조 방법
By fractional distillation of bone oil (bone oil is obtained by destructive distillation of animal bone) and subsequent purification via the corresponding potassium salt; by thermal decomposition of ammonium mucate in glycerol or mineral oil.생산 방법
Pyrrole originally was prepared industrially by fractional distillation of coal tar, bone oil or other protein material, and purified through formation of its potassium derivative (Runge, 1834; Michelman, 1925). Later it was produced by heating ammonium mucate with glycerol or mineral oil (Blicke and Powers, 1927; McElvain and Bollinger, 1941). It is now manufactured by addition of ammonia to either acetylene or butadiene. Good yields of pyrrole also may be obtained from the reaction of ammonia with the corresponding heterocyclic compound (furan) in a vapor-phase process at 480° to 500°C, using alumina as a catalyst (Thompson, 1972) or by catalytic reaction of furan with ammonia over a molybdenum or vanadium oxide catalyst at 350-400°C (Bishop and Denton, 1950).일반 설명
Pyrrole is one of the flavor compounds that is formed in thermally processed foods due to the Maillard reaction.위험도
Moderate fire risk. Toxic by ingestion and inhalation.건강위험
Pyrrole is harmful if swallowed, inhaled, or absorbed through the skin. Its vapor or mist is irritating to the eyes, mucous membranes and upper respiratory tract (Lenga, 1985; Sax, 1984). Although no cases of occupational disease due to pyrrole have been reported, it has a depressant action on the central nervous system and, in severe intoxication, it is injurious to the liver. Tests indicate that it has moderate cumulative toxicity (Parmegianni, 1983).화재위험
Combustible liquid; flash point (closed cup) 39°C (102°F); vapor forms explosive mixtures with air; LEL and UEL values are not available. Heating with strong oxidizers can be violent.공업 용도
Pyrrole is used to a limited extent as a solvent for polymeric esters, but its primary value lies in its function as a chemical intermediate. It is used in the synthesis of non-heterocyclic compounds (Kozikowski, 1984) and its derivatives have been used in the manufacture of dyes, herbicides, perfumes, and as cross-linking agents for curing resins (Thompson, 1972). Derivatives of pyrrole are utilized in pharmaceutical applications, particularly as anti-inflammation drugs and drugs with central nervous system activity, including antihypertensive effects (Sundberg, 1984); and as antimicrobial agents (Freeman, 1975), such as fungicides (Zirngibl, 1983) and bactericides (Bailey and Johnson, 1973; Bailey et al 1973; Sundberg, 1984). Polymers of pyrrole have been used in the preparation of photoconductive materials. The main utility of poly(pyrrole) has been for the modification of electrode surfaces, although numerous other applications can be envisioned (Heilmann and Rasmussen, 1984).Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. Flammable liquid when exposed to heat or flame; can react with oxilzing materials. To fight fire, use foam, CO2, dry chemical. Violent reaction with 2-nitrobenzaldehyde. When heated to decomposition it emits highly toxic fumes of NOx.Purification Methods
Dry pyrrole with NaOH, CaH2 or CaSO4. Fractionally distil it under reduced pressure from CaH2. Store it under nitrogen as it turns brown in air. Redistil it immediately before use. The picrate forms orange-red crystals with m 69o(dec). [Beilstein 20 H 4, 20 I 3, 20 II 3, 20 III/IV 61, 20/5 V 3.]피롤 준비 용품 및 원자재
원자재
준비 용품
Methyl 4-bromopyrrole-2-carboxylate
1-(2-PYRROLIDINOETHYL)PIPERAZINE
Zopiclone
2,5-디메틸피롤
Saquinavir
Zaleplon
PILSICAINIDE
N-(2-CYANOETHYL)PYRROLE
3-Pyrroline
Ketorolac
2-Acetyl pyrrole
Acrivastine
polypyrrole synthesized by electrolytic polymerization
polypyrrole-polyvinyl chloride composite film
글리메피리드
FENPICLONIL
피롤-2-카르복시산
2H-Pyrrol-2-one, 1,3-dihydro-
피롤 공급 업체
글로벌( 379)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xinxiang Hongqi District Houyuan Trading Co.,Ltd | +86-0373-3695376 +86-13937349994 |
HYJM@houyuanjm.com | China | 300 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29811 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21975 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
fandachem@gmail.com | China | 9378 | 55 |
| Jiangsu Qingquan Chemical Co., Ltd. | +86-571-86589381,86589382,86589383 |
sales1@qqpharm.com | CHINA | 154 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29922 | 58 |
| NINGBO INNO PHARMCHEM CO., LTD. | 13867897135 |
sales@nbinno.com | CHINA | 925 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | CHINA | 2933 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22971 | 58 |
피롤 관련 검색:
L-피로글루탐 산 2-히드록시카바졸 하만 하르민 카르바졸 2-피로리딘온,1-에테닐- 피롤리딘 포비돈요오드 폴리비닐피로리돈 N-메틸-2-피롤리디논
1,2,3,4-TETRAHYDRO-9H-PYRIDO[3,4-B]INDOLE
pyrrolo[1,2-a]quinoxaline
2,3,4,4a,9,9a-hexahydro-1H-carbazole
3-Amino-9-ethylcarbazole
N-PHENYLCARBAZOLE HYDROCHLORIDE
2-BENZIMIDAZOLEPROPIONIC ACID
HARMALINE
9H-PYRIDO[3,4-B]INDOLE