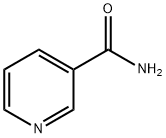
니코틴산아미드 C화학적 특성, 용도, 생산
개요
A white, crystalline powder. It is odorless or nearly so, and has a bitter taste. Its solutions are neutral to litmus. One g dissolves in about 1 mL of water, in about 1.5 mL of alcohol, and in about 10 mL of glycerin.
화학적 성질
Niacinamide is a white crystalline powder
or forms colorless needle-like crystals.
용도
Niacinamide is a nutrient and dietary supplement that is an available form of niacin. Nicotinic acid is pyridine beta-carboxylic acid and nicotinamide, which is another term for niacinamide, is the corresponding amide. It is a powder of good water solubility, having a solubility of 1 g in 1 ml of water. Unlike niacin, it has a bitter taste; the taste is masked in the encapsulated form. Used in fortification of cereals, snack foods, and powdered beverages.
정의
ChEBI: A pyridinecarboxamide that is pyridine in which the hydrogen at position 3 is replaced by a carboxamide group.
일반 설명
Vitamin B
3 was formerly called nicotinic acid; however, the term niacin is now preferred to avoid any confusion with the alkaloid, nicotine. Niacinamide, also known as nicotinamide, refers to the amide derivative of niacin that is equivalent in vitamin activity. Some texts use niacin to refer to nicotinic acid, niacinamide, and any derivatives with vitamin activity comparable to niacin. Furthermore, research and chemistry-based resources use the terms nicotinic acid and nicotinamide; whereas pharmacy resources use niacin and niacinamide.
공기와 물의 반응
Water soluble.
반응 프로필
An amine and amide. Acts as a weak base in solution. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Clinical Use
Niacin is used in the treatment of niacin deficiency, which is referred to as pellagra (from the Italian, pelle for “skin” and agra for “dry”). The major systems affected are the gastrointestinal tract (diarrhea, enteritis and stomatitis), the skin (dermatitis), and the CNS (generalized neurological deficits including dementia). Pellagra has become a rare condition in the United States and other countries that require or encourage enrichment of wheat flour or fortification of cereals with niacin. Because the nucleotide form can be synthesized in vivo from tryptophan, pellagra is most often seen in areas where the diet is deficient in both niacin and tryptophan. Typically, maize (corn)-based diets meet this criteria. Niacin deficiency can also result from diarrhea, cirrhosis, alcoholism, or Hartnup disease. It is interesting that niacin deficiency can also, rarely, result from vitamin B
6 deficiency (see Vitamin B
6 section). Niacin, but not niacinamide, is also one of the few vitamins that are useful in the treatment of diseases unrelated to deficiencies.
Safety Profile
Nicotinamide is a safe and inexpensive compound with negligible side effects. It is well tolerated even in doses of 1g/day to 3g/day.There are no reports of teratogenicity with nicotinamide. Minor side effects include nausea, vomiting, headache, fatigue. It does not cause vasodilatory side effects like flushing, alteration in blood pressure, body temperature or pulse as seen with niacin.In topical formulation, it does not cause skin irritation, photosensitization in concentrations of 0.0001% to 4%.
잠재적 노출
Used as a dietary supplement and
food additive.
신진 대사
Nicotinamide is ingested in food as part of pyridine nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) in plant and animal tissues. After the co?enzymes have separated, nicotinamide is absorbed almost completely in the small intestine. After absorption, nicotinamide is stored as NAD in the liver and excretion occurs via kidneys. Tryptophan is converted to nicotinamide through kynurenine?anthranilate pathway in the liver. Tryptophan can thus satisfy the requirement for dietary nicotinic acid.
Purification Methods
Crystallise niacin from *benzene. It has solubility in g/ml: H2O (1), EtOH (0.7) and glycerol (0.1). [Methods in Enzymology 66 23 1980, UV: Armarego Physical Methods in Heterocyclic Chemistry (Ed Katritzky, Academic Press) Vol III 83 1971, Beilstein 22 III/IV 389, 22/2 V 80.]
비 호환성
Combustible solid; dust may form explosive
mixture with air. Amides are incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.); contact may
cause fires or explosions. Keep away from alkaline materials,
strong bases, strong acids, oxoacids, epoxides.
니코틴산아미드 준비 용품 및 원자재
원자재
준비 용품