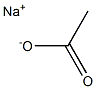
Sodium acetate
- CAS No.
- 127-09-3
- Chemical Name:
- Sodium acetate
- Synonyms
- Sodium acetate anhydrous;Anhydrous sodium acetate;BUFFER SOLUTION;Natriumacetat;sodium ethanoate;SODIUM ACETATE BUFFER;NA ACETATE;sodiiacetas;femanumber3024;natriumaceticum
- CBNumber:
- CB1230044
- Molecular Formula:
- C2H3NaO2
- Molecular Weight:
- 82.03379
- MDL Number:
- MFCD00012459
- MOL File:
- 127-09-3.mol
- MSDS File:
- SDS
| Melting point | >300 °C (dec.)(lit.) |
|---|---|
| Density | 1.01 g/mL at 20 °C |
| refractive index | 1.4640 |
| FEMA | 3024 | SODIUM ACETATE |
| Flash point | >250 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 3 M at 20 °C, clear, colorless |
| form | powder |
| pka | 4.756[at 20 ℃] |
| Specific Gravity | 1.45 |
| color | white |
| PH | 7.87(1 mM solution);8.33(10 mM solution);8.75(100 mM solution);9.04(1000 mM solution) |
| Odor | Slight acetic acid |
| PH Range | 8.5 - 9.9 at 246 g/l at 25 °C |
| Odor Type | odorless |
| Water Solubility | 500 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| λmax |
λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,8571 |
| Boiling point | >400°C(decomposition) |
| BRN | 3595639 |
| Stability | Stable. Incompatible with strong oxidizing agents, halogens. Moisture sensitive. |
| InChIKey | VMHLLURERBWHNL-UHFFFAOYSA-M |
| LogP | -3.72 |
| FDA 21 CFR | 184.1721; 582.1721; 173.310; 182.70 |
| CAS DataBase Reference | 127-09-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NVG71ZZ7P0 |
| ATC code | B05XA08 |
| NIST Chemistry Reference | Sodium ethanoate(127-09-3) |
| EPA Substance Registry System | Sodium acetate (127-09-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H320 | |||||||||
| Precautionary statements | P264-P337+P313-P305+P351+P338 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AJ4300010 | |||||||||
| F | 3 | |||||||||
| Autoignition Temperature | 607 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29152200 | |||||||||
| Toxicity | LD50 orally in Rabbit: 3530 mg/kg LD50 dermal Rabbit > 10000 mg/kg | |||||||||
| NFPA 704 |
|
Sodium acetate price More Price(137)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W302406 | Sodium Acetate Anhydrous >99%, FCC, FG | 127-09-3 | 1kg | $97.3 | 2023-06-20 | Buy |
| Sigma-Aldrich | W302406 | Sodium Acetate Anhydrous >99%, FCC, FG | 127-09-3 | 10Kg | $223 | 2023-06-20 | Buy |
| Sigma-Aldrich | W302406 | Sodium Acetate Anhydrous >99%, FCC, FG | 127-09-3 | 25kg | $520 | 2023-06-20 | Buy |
| Sigma-Aldrich | 32319 | Sodium acetate puriss. p.a., ACS reagent, reag. Ph. Eur., anhydrous | 127-09-3 | 500G | $105 | 2023-06-20 | Buy |
| Sigma-Aldrich | 32319 | Sodium acetate puriss. p.a., ACS reagent, reag. Ph. Eur., anhydrous | 127-09-3 | 1KG | $185 | 2023-06-20 | Buy |
Sodium acetate Chemical Properties,Uses,Production
Description
Sodium acetate (CH3COONa) is the sodium salt of acetic acid. It appears as a colorless deliquescent salt with a wide range of applications. In industry, it can be used in textile industry to neutralize sulfuric acid waste streams and as a photoresist upon using aniline dyes. In concrete industry, it can be used as a concrete sealant to mitigate the water damage. In food, it can be used as a seasoning. It can also be used as a buffer solution in lab. In addition, it is also used in heating pads, hand warmers and hot ice. For laboratory use, it can be produced by the reaction between acetate with the sodium carbonate, sodium bicarbonate and sodium hydroxide. In industry, it is prepared from the glacial acetic acid and sodium hydroxide.
Chemical Properties
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.
Chemical Properties
Sodium acetate, CH3COONa, also abbreviated NaOAc , also sodium ethanoate, is the sodium salt of acetic acid, was made by the reaction of acetic acid with sodium carbonate. It is soluble in water but less so in alcohol. This colourless salt has a wide range of uses. Sodium acetate was used as a pH modifier for toning baths.
Chemical Properties
Sodium acetate is odorless or has a faint acetous odor. It effloresces in warm, dry air.
Physical properties
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.
Occurrence
Acetic acid or acetates are present in most plant and animal tissues in small, but detectable amounts
Uses
Sodium Acetate, Anhydrous is a source of acetic acid obtained as a granular powder. it has a solubility of 1 g in 2 ml of water.
Uses
Sodium acetate is a mordant in dyeing. Other applications are in photography, as an additive to food, in purification of glucose, in preservation of meat, in tanning, and as a dehydrating agent. In analytical chemistry it is used to prepare buffer solution.
Sodium acetate can be used to preserve processed meats and it is often used in combination with other acid based preservatives like lactates and propionates. The typical inclusion level is 0.2 to 0.5%. Sodium acetate is also used in salad dressings and ready-to-eat meals.
Uses
Used as buffers.
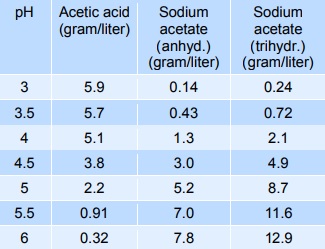
Acidity regulation (buffering)
Sodium acetate mixed with acetic acid forms a pH buffer, which can be used to stabilise the pH of foods in the pH-range from 3 to 6. The table below gives indicative values of the composition needed to give a certain pH. The mixtures below can be diluted at least 10 times with minimum effect on pH, however, the stability decreases.
Preparation
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.
NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
Definition
A white solid prepared by the neutralization of ethanoic acid with either sodium carbonate or sodium hydroxide. Sodium ethanoate reacts with sulfuric acid to form sodium hydrogensulfate and ethanoic acid; with sodium hydroxide it gives rise to sodium carbonate and methane. Sodium ethanoate is used in the dyeing industry.
Application
2 - 1 - Industrial
Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams, and as a photoresist while using aniline dyes. It is also a pickling agent in chrome tanning, and it helps to retard vulcanization of chloroprene in synthetic rubber production. In processing cotton for disposable cotton pads, sodium acetate is used to eliminate the buildup of static electricity.
2 - 2 - Concrete longevity
Sodium acetate is used to reduce the damage water can potentially do to concrete by acting as a concrete sealant, while also being environmentally benign and cheaper than the epoxy alternative that is usually employed for sealing concrete against water permeation.
2 - 3 - Food
Sodium acetate may be added to foods as a seasoning. It may be used in the form of sodium diacetate — a 1:1 complex of sodium acetate and acetic acid, given the E-number E262. A frequent use is to impart a salt and vinegar flavor to potato chips.
2 - 4 - Buffer solution
As the conjugate base of acetic acid, a solution of sodium acetate and acetic acid can act as a buffer to keep a relatively constant pH.
2 - 5 - Heating pad
Sodium acetate is also used in consumer heating pads or hand warmers and is also used in hot ice. Sodium acetate trihydrate crystals melt at 58.4°C , (to 58°C ) dissolving in their water of crystallization. When they are heated to around 100°C, and subsequently allowed to cool, the aqueous solution becomes supersaturated. This solution is capable of cooling to room temperature with out forming crystals.
Synthesis
For laboratory use, sodium acetate is very inexpensive, and is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid (ethanoic acid) with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda (sodium bicarbonate) and vinegar.
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Industrially, sodium acetate is prepared from glacial acetic acid and sodium hydroxide.
CH3COOH + NaOH → CH3COONa + H2O.
Reactions
Sodium acetate can be used to form an ester with an alkyl halide such as bromo ethane:
CH3COONa + Br CH2CH3→ CH3COOCH2CH3+ NaBr
Caesium salts catalyze this reaction.
General Description
Sodium Acetate is reported to inhibit the growth of Listeria monocytogenes.
Reactivity Profile
When sodium acetate reacts with strong acids, irritating, noxious vapors of acetic acid are usually produced. Sodium acetate is sufficiently basic to catalyze the violent polymerization of diketene, perhaps as well as other reactive dimers that are susceptible to polymerization in the presence of a mild base.
Flammability and Explosibility
Nonflammable
Biological Activity
Commonly used laboratory reagent
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. A skin and eye irritant. Migrates to food from packagmg materials. Violent reaction with F2, m03, diketene. When heated to decomposition it emits toxic fumes of Na2O.
Synthesis
Acetic acid plus sodium bicarbonate makes sodium acetate plus carbonic acid. Produced by the neutralization of acetic acid with sodium bicarbonate, or by treating calcium acetate with sodium sulfate and sodium bicarbonate.
Purification Methods
Crystallise it from acetic acid and keep it under vacuum for 10hours at 120o. Alternatively, it is crystallised from aqueous EtOH, as the trihydrate. This material can be converted to anhydrous salt by heating slowly in a porcelain, nickel or iron dish, so that the salt liquefies. Steam is evolved and the mass again solidifies. Heating is now increased so that the salt melts again. (NB: if it is heated too strongly, the salt can char; avoid this.) After several minutes, the salt is allowed to solidify and is cooled to a convenient temperature (in a desiccator) before being powdered and bottled. The water content should now be less than 0.02%. [Beilstein 2 II 113, 2 III 184, 2 IV 109.]
Sodium acetate Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 | sales3257@jskolod.com | China | 130 | 60 |
| Weijer International Trade (Hebei) Co., Ltd | +8619556593063 | China | 991 | 58 | |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6123 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2180 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29811 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21975 | 55 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 | fandachem@gmail.com | China | 9378 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2990 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| HEBEI YINGONG NEW MATERIAL TECHNOLOGY CO.,LTD | +86-19933070948 +86-19930599843 | claire@hbyingong.com | China | 945 | 58 |
Related articles
- Sodium Acetate: Preparation, Reactions and Applications
- Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wi....
- Apr 12,2023
- Practical Applications of Sodium Acetate
- Sodium acetate salt, or simply sodium acetate, has many practical uses. It is the conjugate base of a weak acid, meaning that ....
- Nov 12,2019
View Lastest Price from Sodium acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-10 | Sodium acetate
127-09-3
|
US $30.00 / KG | 1KG | 99.9% | 1000kg/month | Shanxi Lianxu New Material Co., LTD | |
 |
2023-08-10 | Sodium acetate
127-09-3
|
US $126.00 / kg | 100kg | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2023-07-31 | Sodium acetate
127-09-3
|
US $1.00 / kg | 10kg | 99% | 20tons | Hebei Linwo New Material Technology Co., LTD |
-

- Sodium acetate
127-09-3
- US $30.00 / KG
- 99.9%
- Shanxi Lianxu New Material Co., LTD
-

- Sodium acetate
127-09-3
- US $126.00 / kg
- 99.99%
- Hebei Dangtong Import and export Co LTD
-

- Sodium acetate
127-09-3
- US $1.00 / kg
- 99%
- Hebei Linwo New Material Technology Co., LTD
127-09-3(Sodium acetate)Related Search:
1of4